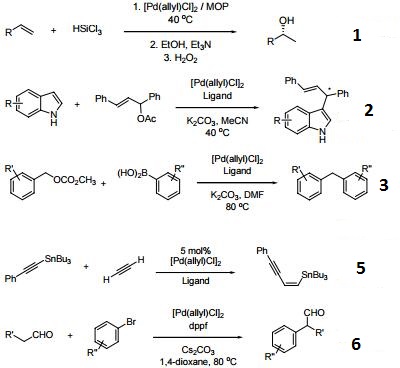
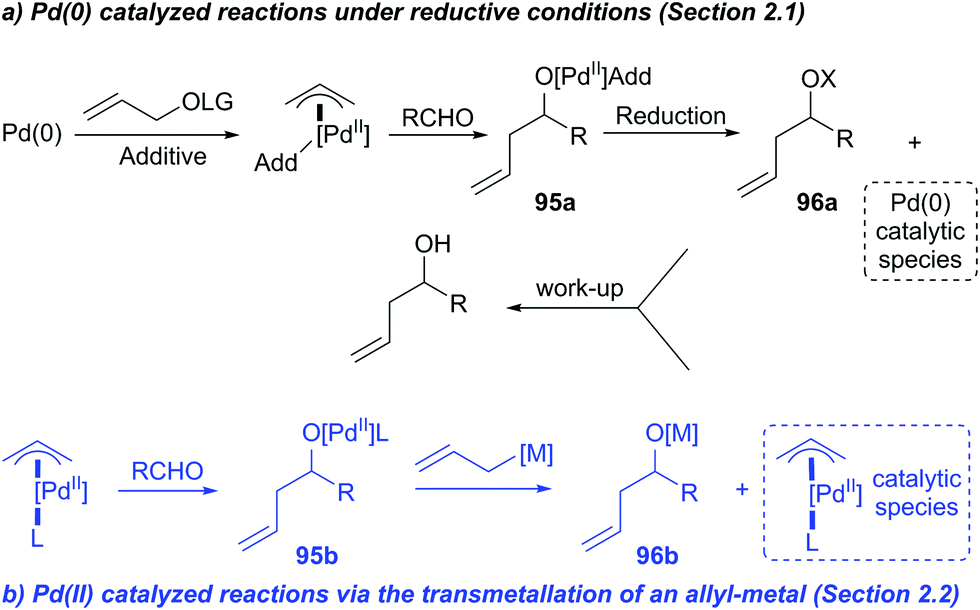
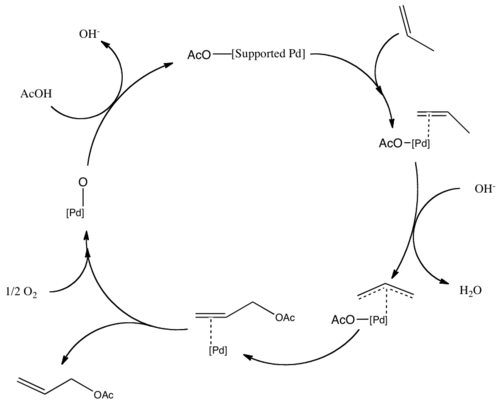
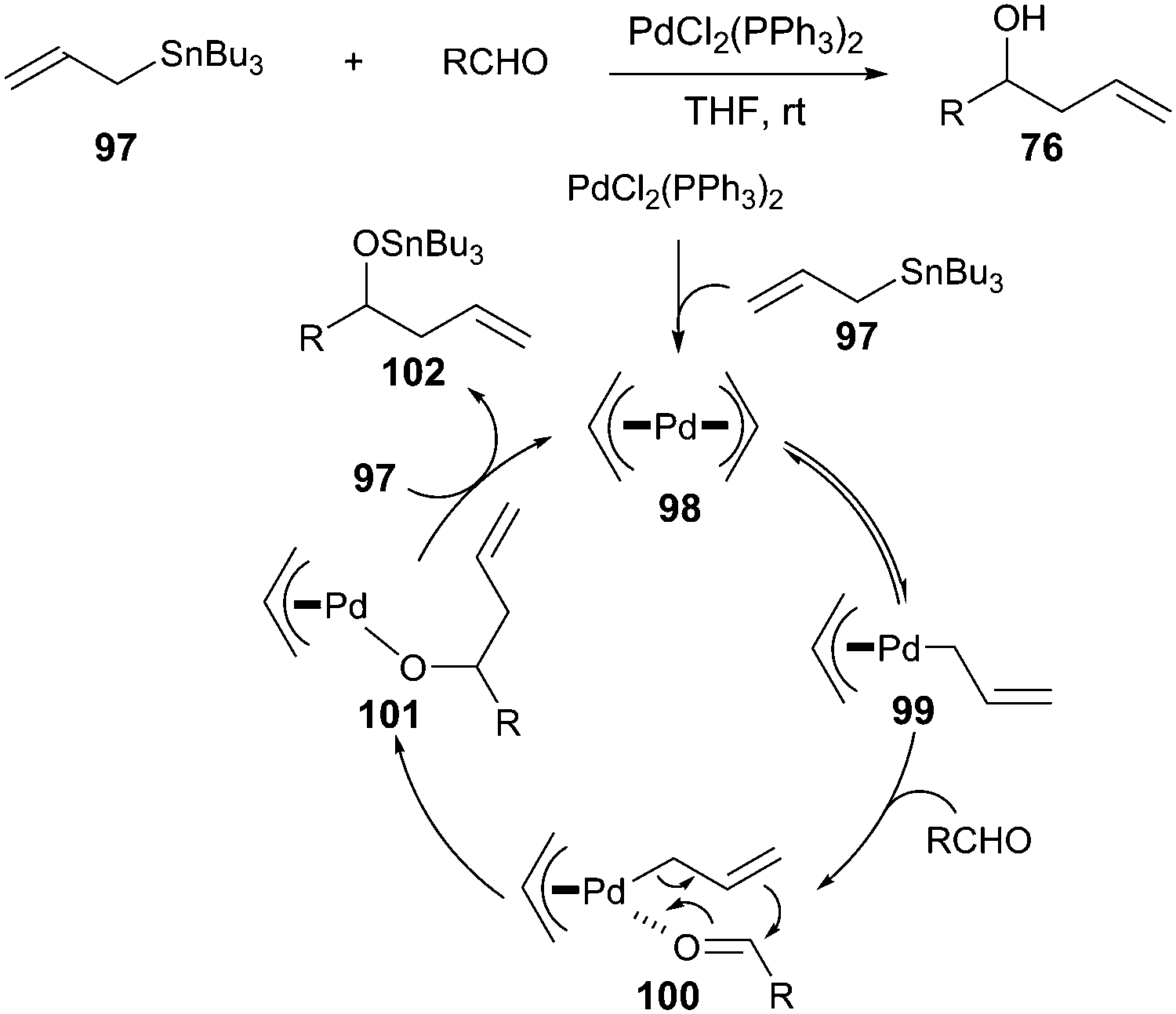
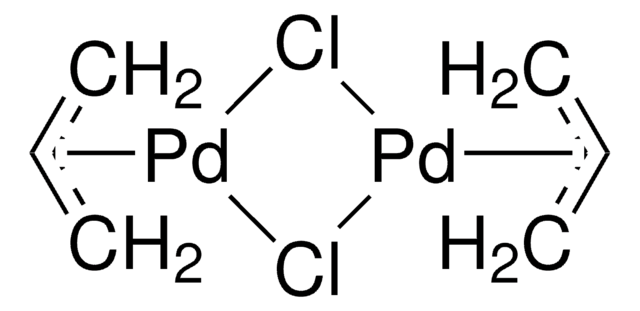
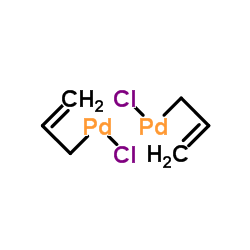
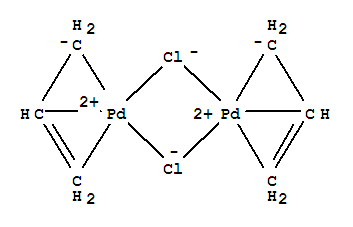
![Inorganic Syntheses] Inorganic Syntheses Volume 19 || (η3-Allyl)Palladium(II) Complexes - [PDF Document] Inorganic Syntheses] Inorganic Syntheses Volume 19 || (η3-Allyl)Palladium(II) Complexes - [PDF Document]](https://reader011.staticloud.net/reader011/html5/20190220/5750931f1a28abbf6bad5898/bg1.png)
Inorganic Syntheses] Inorganic Syntheses Volume 19 || (η3-Allyl)Palladium(II) Complexes - [PDF Document]

Steric Effects in Enantioselective Allylic Alkylation Catalysed by Cationic(η3‐Allyl)palladium Complexes Bearing Chiral Pyridine‐Aziridine Ligands - Ferioli - 2005 - European Journal of Organic Chemistry - Wiley Online Library
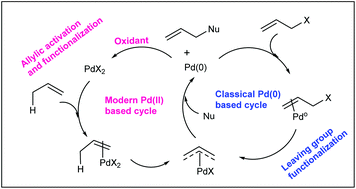
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing)

Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect
![PDF] Confirming the existence of π-allyl-palladium intermediates during the reaction of meta photocycloadducts with palladium(II) compounds. | Semantic Scholar PDF] Confirming the existence of π-allyl-palladium intermediates during the reaction of meta photocycloadducts with palladium(II) compounds. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/e3170611736068557037b936b6641e36bc061f7e/4-Figure2-1.png)
PDF] Confirming the existence of π-allyl-palladium intermediates during the reaction of meta photocycloadducts with palladium(II) compounds. | Semantic Scholar

Palladium‐Catalyzed Electrophilic Allylation Reactions via Bis(allyl) palladium Complexes and Related Intermediates - Szabó - 2004 - Chemistry – A European Journal - Wiley Online Library

Alfa Aesar™ Allyl(chloro)(di-tert-butylneopentylphosphine)palladium(II), may cont. up to ca 5% toluene 1g Organic phosphines and derivatives | Fisher Scientific
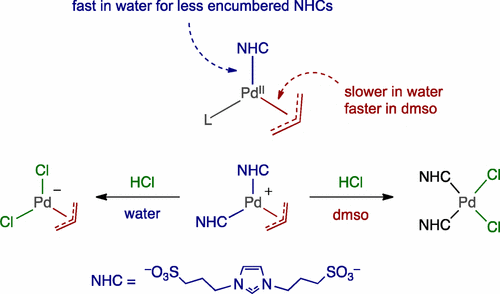
Aqueous-Phase Chemistry of η3-Allylpalladium(II) Complexes with Sulfonated N-Heterocyclic Carbene Ligands: Solvent Effects in the Protolysis of Pd–C Bonds and Suzuki–Miyaura Reactions - Organometallics - X-MOL

Mechanism of allyl deprotection through catalytic palladium π-allyl... | Download Scientific Diagram

Stereochemistry of the palladium-catalyzed allylic substitution: the syn-anti dichotomy in the formation of (π-allyl)palladium complexes and their equilibration - ScienceDirect

Highly enantioselective palladium-catalyzed umpolung allylation of aldehydes - Chemical Science (RSC Publishing)
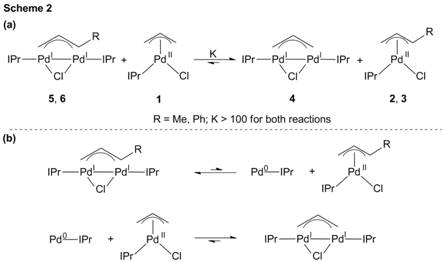
Report: Palladium(I) and Nickel(I) Bridging Allyl Dimers for the Catalytic Functionalization of CO2 (58th Annual Report on Research Under Sponsorship of The American Chemical Society Petroleum Research Fund)

Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications
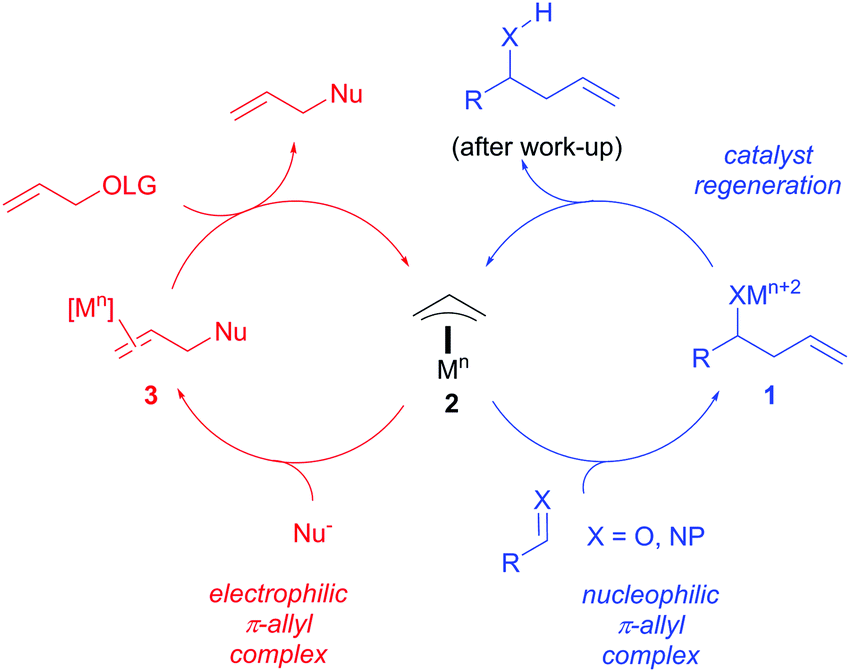
A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions
![Alfa Aesar™ Allyl(chloro)[di-tert-butyl(4-dimethylaminophenyl)phosphine] palladium(II) 1g Phenylmethylamines | Fisher Scientific Alfa Aesar™ Allyl(chloro)[di-tert-butyl(4-dimethylaminophenyl)phosphine] palladium(II) 1g Phenylmethylamines | Fisher Scientific](https://assets.fishersci.com/TFS-Assets/CCG/Chemical-Structures/chemical-structure-cas-1235509-04-2.jpg-650.jpg)
Alfa Aesar™ Allyl(chloro)[di-tert-butyl(4-dimethylaminophenyl)phosphine] palladium(II) 1g Phenylmethylamines | Fisher Scientific

Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect
A Mechanistic Study of Direct Activation of Allylic Alcohols in Palladium Catalyzed Amination Reactions
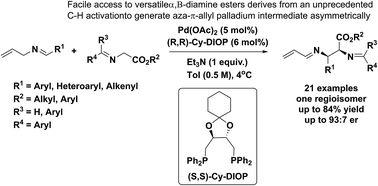
Pd-catalyzed asymmetric allylic alkylations via C–H activation of N-allyl imines with glycinates - Chemical Science (RSC Publishing)